Wherible Age-Tech and Alzheimer’s Disease
According to the National Institutes of Health (NIH), National Institute on Aging and the National Institute of Neurological Disorders and Stroke (NINDS) the advantages of using digital (technology) endpoints in AD clinical trials are numerous…
Read more...
Individuals with ADRD can have many physiological changes that lead to a variety of symptoms and changes in function that negatively impact quality of life but may be difficult to accurately monitor and track through standard clinical outcome assessments. Examples include sleep disorders, such as idiopathic REM sleep behavior disorder (RBD) in LDB, and insomnia and daytime sleepiness in FTD. Likewise, mobility, autonomic dysfunction such as orthostatic hypotension leading to postural instability and falls, as well as gastrointestinal issues, all negatively impact quality of life, but are difficult to accurately track and quantify when relying on self-report or caregiver reports. Use of digital health technologies (DHTs) to enable monitoring in clinical trials as exploratory or secondary endpoints is rapidly increasing. Advantages of using digital endpoints in clinical trials are numerous, including enabling higher frequency longitudinal data collection in real world conditions and reducing the burden of participation in clinical trials, which can increase participant recruitment and retention. However, like all biomarkers and clinical assessments, demonstrating utility and reliability requires extensive fit-for-purpose validation. As described in the FDA’s draft guidance “Digital Health Technologies for Remote Data Acquisition in Clinical Investigations”, evidence must include verification that the device can accurately, and reliability measure the physiological, functional or behavioral characteristics that make up the biomarker(s) or assessments of interest, and also establish the statistical relationship between the changes in the biomarker or assessment with a clinically meaningful change in how the patient feels, functions, or survives. Although Digital Health Technologies (DHTs) are broadly defined as “a system that uses computing platforms, connectivity, software, and/or sensors for healthcare and related uses”, the focus of funding opportunities is on developing digital biomarkers and performance/functional assessments from data generated from wearable sensor-based devices.
In 2011, the National Alzheimer’s Project Act (NAPA) allocated resources “to prevent and effectively treat Alzheimer’s by 2025.” Since then, the National Institute on Aging (NIA) and the National Institute of Neurological Disorders and Stroke (NINDS) have held multiple research summits to assess the needs and opportunities relevant to this goal for Alzheimer’s Disease (AD) and Alzheimer’s Disease Related Dementias (ADRD). In particular, the NINDS has convened expert panels to discuss and to recommend research priorities for advancing the state-of-the-science for the Alzheimer’s disease (AD) and Alzheimer’s disease-related dementias (ADRD) by 2025. ADRD are defined as Frontotemporal Degeneration (FTD), Vascular Contributions to Cognitive Impairment and Dementia (VCID), Lewy Body Dementias (LBD) and Multiple Etiology Dementias (MED).
Edge Technology in Early Detection of ADRD
Wherible is not only a home safety solution for families with vulnerable seniors and adults at home, alone, it has significant application in the monitoring and early detection of chronic diseases like Dementia, Parkinson’s, Diabetes, Heart Disease and more, through its Edge Device Technology. The Edge devices monitor for Activities of Daily Living (ADL’s), movement, and gait and gait speed, for example.
Read more...
The National Institutes of Health and medical researchers are interested in capturing digital data from wearable devices. Data from these are considered digital biomarkers and are a ‘hot’ new emerging technology, that has the potential to provide supportive digital data and insights not only on earlier identification and disease progression, but if new treatments are effective, or not during a clinical trial, for example.
How Do Edge Devices Work?
-
Wherible Devices use AI and Machine Learning (ML) to collect and formulate baseline movement ADL data
-
Personal unique movement data, we call their ‘signature’ are sent to the Wherible cloud platform
-
The cloud platform formulates personalized algorithms which are sent back to the Wherible Edge device
-
The Wherible Edge device is optimized to continually capture ADL’s and build upon the data every day
-
The AI/ML is a revolving loop of data cycling back to the cloud, essentially validating the accuracy of the data
-
If the patterns of ADL’s change the Wherible platform identifies those changes
-
Research has identified known physiological parameters of Dementia, and our technology can detect it
AI and ML Feedback Loop between Edge Devices and Wherible Cloud
The Future Game Changer In ADRD and Chronic Disease Clinical Trials
Wherible researchers saw clearly that to use Edge device machine learning novel algorithms, the following parameters were required:
-
Fit-for-purpose built Edge devices
-
Proprietary age-specific body movement data capture vs. a basic fitness tracker
-
Edge devices must be continually tuned and validated by cloud platform
-
New age-specific data continually learns and creates novel unique personalized algorithms
-
Must include contextual data – if no wearable ADL monitoring device is worn
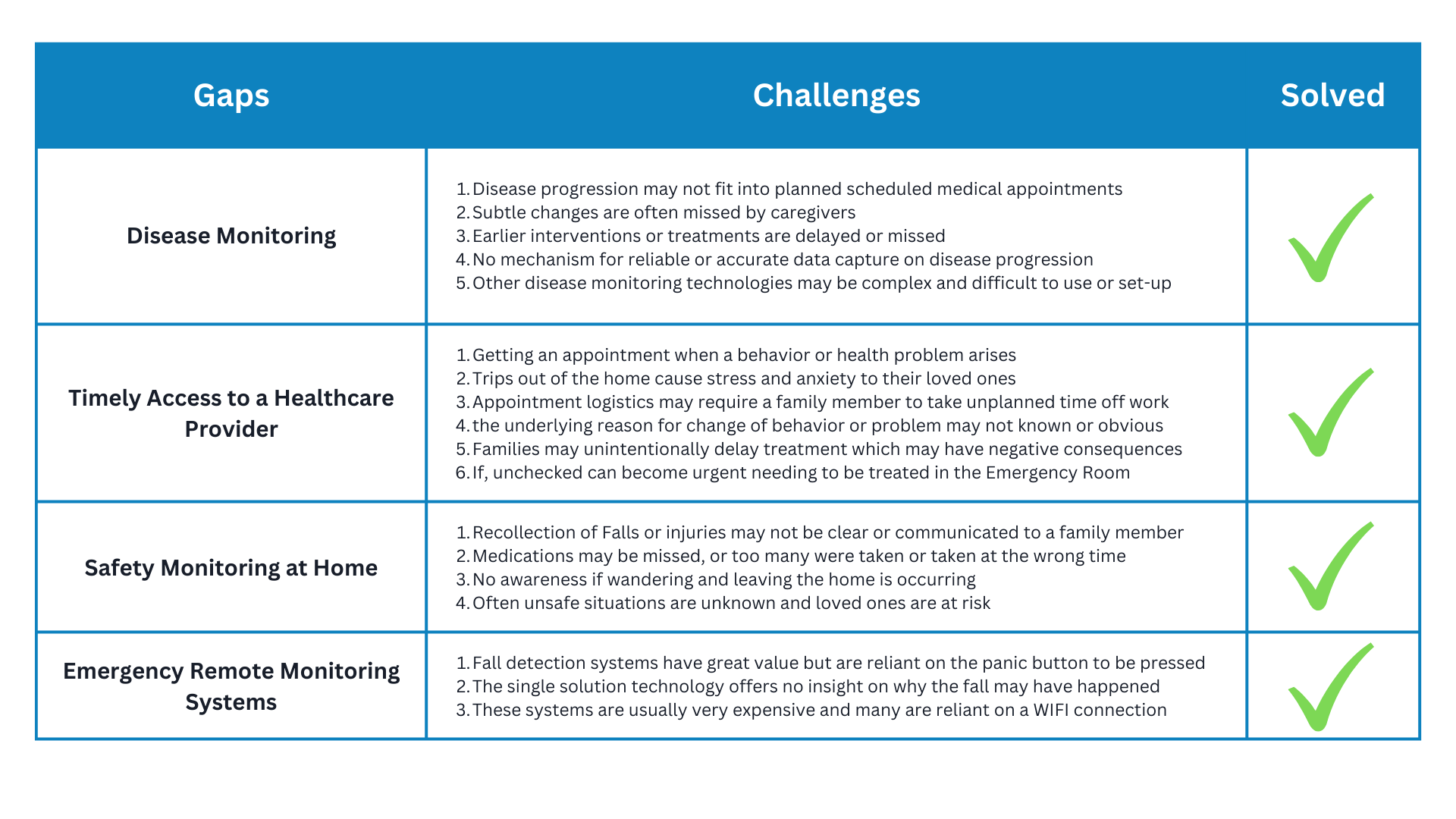
Wherible Helps Fill the Gaps in Dementia Disease Monitoring, and Memory Recall
Remote safety monitoring of Dementia patients at home provides the healthcare team insight into the early onset and detection of memory loss, the progression of their disease, and treatment efficacy.
Additionally, it’s a great solution when those with dementia can’t tell us, if they fell or took their medications, don’t feel well, have a bladder infection, or if they left their home.